
News
JPM 2026 | Sino Biopharm Leads in siRNA and Tumor Innovation Platforms, M&A Integration Unleashes Long-Term Growth Momentum
Release Date: 2026-01-16
On January 15, local time, Sino Biopharm (1177.HK) attended the 44th J.P. Morgan Healthcare Conference and gave a systematic presentation on the company's strategic M&A, technology platforms, and innovative pipeline, showcasing its steady pace of accelerating innovation and expansion to the global market. Mr. Hsin Tse, Executive Director and Senior Vice President of Sino Biopharm, as well as Dr. Qin Ying, founder of the Group's wholly-owned subsidiary LaNova Medicines, Dr. Cui Kunyuan, founder of Hygieia, and Mr. Yang Zhenchang, Head of Overseas Business, attended the conference and delivered speeches.

Building Differentiated Competitiveness in small interfering RNA (siRNA), Leading a New Era of "Once-Yearly" Dosing
Hygieia is a leading biopharmaceutical company focused on the R&D of innovative siRNA drugs. It has six major siRNA delivery technology platforms covering liver (single/dual target) and extra-liver targeting of multiple tissues such as nerves, lungs, kidneys, and fat, and has established an R&D pipeline of over 20 items covering the three major chronic disease areas of weight loss and metabolism, cardiovascular and cerebrovascular disorders, and the nervous system. Sino Biopharm's full acquisition of Hygieia is a strategic milestone transaction of "platform + pipeline" that not only rapidly builds Sino Biopharm's leading differentiated competitiveness in the siRNA track but also marks the company's accelerated expansion into the trillion-dollar chronic disease market.
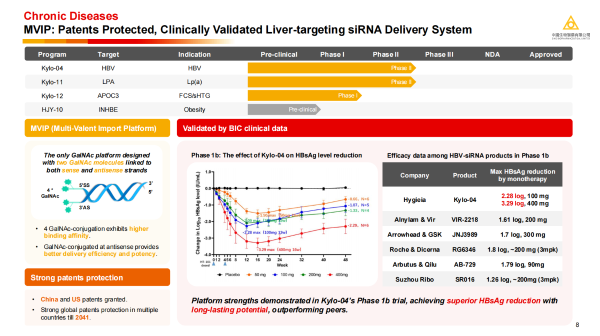
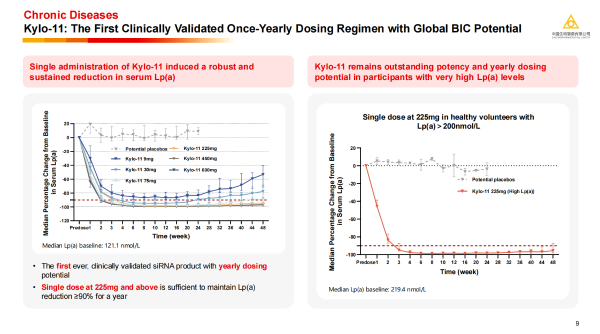
Dr. Cui Kunyuan introduced that the MVIP liver-targeting platform is the world's first and currently only siRNA delivery technology platform clinically validated to achieve "once-yearly" dosing. Its unique design involves attaching four GalNAc (N-acetylgalactosamine) molecules to both the sense and antisense strands of the siRNA, demonstrating higher targeting affinity and delivery efficiency. The platform has been granted patents in both China and the US, overcoming the key "bottleneck" restrictions faced by domestic small nucleic acid in out-licensing deals. · Kylo-11: A small nucleic acid product developed based on this platform for treating high Lp(a) levels. Clinical study data show that a single dose at 225 mg can induce a reduction in serum Lp(a) levels by over 90%, with the effect lasting up to one year; even in subjects with extremely high baseline Lp(a) levels,
· Kylo-11 can achieve and maintain a reduction of about 90%, demonstrating Best-in-Class (BIC) potential globally, and is expected to bring revolutionary changes to the prevention and treatment of cardiovascular disorders.
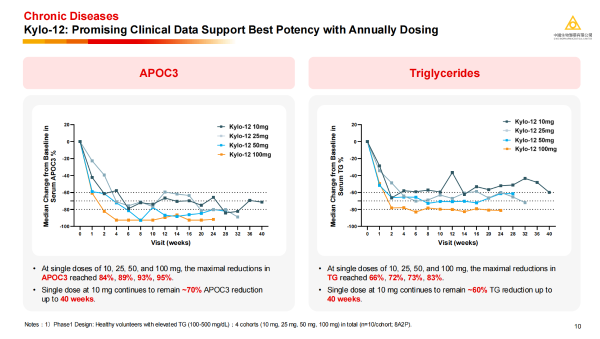
· Kylo-12: Developed based on this platform to target APOC3, Kylo-12 has also shown strong clinical potential in the treatment of severe hypertriglyceridaemia (sHTG). A single dose at 10 mg can maintain a reduction of about 70% in APOC3 and about 60% in triglycerides (TG) after 40 weeks, and the efficacy shows significant dose dependence, indicating its potential to achieve significant benefits with once-yearly dosing.
· Kylo-04: Developed based on the MVIP platform, Kylo-04 achieved a superior reduction in HBsAg (hepatitis B surface antigen) compared to its peers in a Phase 1b clinical trial for the treatment of hepatitis B (HBV), with a reduction of up to 3.29 log in the 400 mg dose group, demonstrating excellent efficacy, durability, and the high delivery efficiency of the MVIP platform.
The DDP dual-target delivery platform can simultaneously deliver dual-target siRNAs, solving the key problem of "1+1<2" in traditional dual-target drug therapy. It is not limited to intra-hepatic delivery platforms and can be combined with other extra-hepatic delivery platforms targeting different tissues to develop treatments for complex or refractory diseases mediated by multiple targets and mechanisms, possessing significant technological disruption potential.
The NSDP nerve-targeting platform prospectively lays out delivery in the field of nervous system diseases, filling the unmet clinical needs for the CNS (central nervous system) and PNS (peripheral nervous system). Preclinical data show that HJY-02, for the treatment of Alzheimer's disease, can efficiently deliver siRNA to key areas of the brain (such as the hippocampus), selectively inhibiting the expression of the target gene, and shows potential for once-yearly dosing. It is expected to enter the clinical stage in 2026, helping to expand the Group's R&D pipeline to a broader range of disease areas.
Securing a Technological Stronghold in the Tumor Microenvironment, FIC/BIC Pipeline Accelerates Commercialization Process
In the field of oncology, Sino Biopharm completed the full acquisition of LaNova Medicines in July 2025. LaNova Medicines is a research-driven biotechnology company focusing on the tumor microenvironment (TME) and immunotherapy (IO) fields, and is dedicated to developing FIC/BIC innovative drugs. In just six years, it has established 8 clinical-stage and over 20 preclinical projects. LM-305 and LM-299 have been successively out-licensed to global multinational pharmaceutical companies MSD and AstraZeneca.
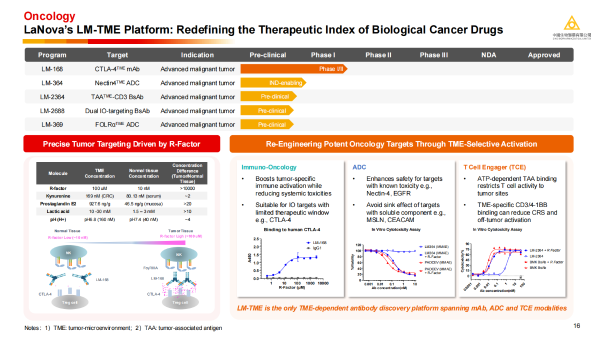
Dr. Qin Ying introduced that the core competitiveness of LaNova Medicines lies in its unique LM-TME platform. This platform aims to solve a fundamental challenge in the field of tumor biologics: how to reduce systemic toxicity while maintaining potent killing effects. The LM-TME platform utilizes the characteristic that the concentration of certain biochemical factors (R-factors) in tumor tissue is much higher than in normal tissue to design TME-selectively activated antibody drugs, thereby "revitalizing" targets like CTLA-4 that have historically been limited by toxicity. Furthermore, LM-TME is currently the only known TME-dependent antibody discovery platform that covers three drug formats: monoclonal antibodies (mAb), Antibody-Drug Conjugates (ADCs), and T-cell Engagers (TCEs). It is a powerful engine for developing differentiated innovative biologics.
· LM-302: A CLDN18.2 ADC with global First-in-Class (FIC) potential, has received Breakthrough Therapy Designation for the treatment of advanced or metastatic gastric or gastroesophageal junction adenocarcinoma. LM-302 is scheduled to initiate a Phase 3 clinical trial in 1L gastric cancer in March 2026 and is expected to submit a marketing application to the National Medical Products Administration (NMPA) for ≥3L gastric cancer in mid-2026. The latest research data for the 1L pancreatic cancer are expected to be read out this year.
· LM-108: A CCR8 monoclonal antibody with FIC potential, has received two Breakthrough Therapy Designations. Its unique mechanism of action can overcome resistance to PD-1/PD-L1 inhibitors, providing a new treatment option for patients who have failed immunotherapy. LM-108 is scheduled to start a Phase 3 clinical trial in 2L gastric cancer during the same period and will announce its Phase 1/2 clinical data in 1L gastric cancer and 1L pancreatic cancer at the end of the year.
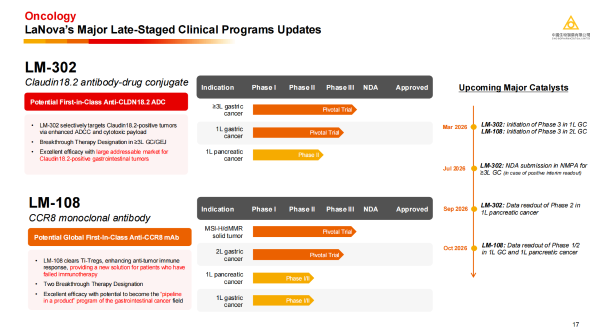
These clear value inflection points indicate that LaNova Medicines is about to enter a period of commercial harvest.
Empowered by AI Small Molecules and the OAPD® Platform, Endogenous Innovative Pipeline Achieves Remarkable Results
In addition to achieving leapfrog development through M&A, Sino Biopharm has also demonstrated strong endogenous R&D capabilities. The company highlighted the latest progress in its AI-assisted small molecule drug and OAPD® (Orally Available Protein Degrader) drug discovery platforms.
The OAPD® platform was independently built by Chia Tai Tianqing and has global independent intellectual property rights. It significantly accelerates the discovery of protein degraders with the help of AI molecular generation technology. Through precise design and optimization, it not only exhibits excellent degradation activity and superior oral bioavailability, but can also significantly reduce safety issues caused by immunomodulatory activity.
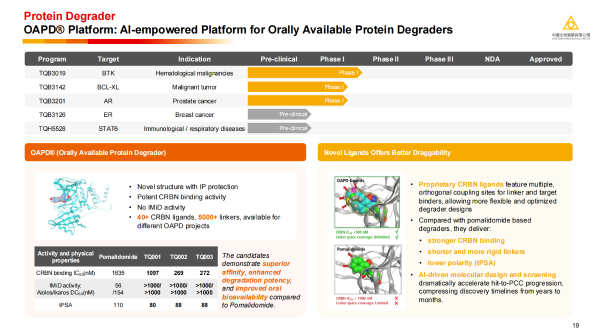
· TQB3019: An oral, BTK-targeting Proteolysis-targeting chimera (PROTAC) drug with an oral bioavailability of up to 40% in animal models. In the first dosing cohort of the Phase 1 study, 4 of 5 patients achieved partial responses across mantle cell lymphoma (MCL), follicular lymphoma (FL), and chronic lymphocytic leukaemia (CLL). Pharmacodynamic analyses showed near-complete BTK degradation.
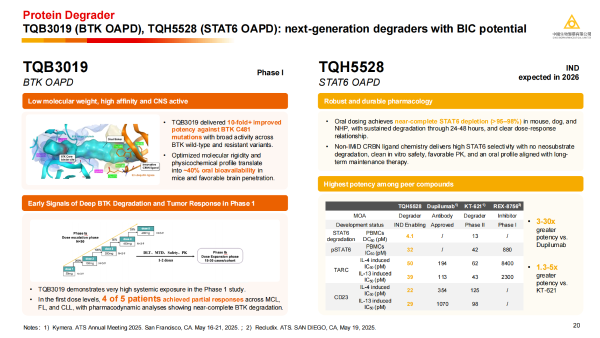
· TQH5528: Preclinical studies show that STAT6 in key tissues is almost completely degraded even at very low oral doses, positioning it as a potential next-generation oral immunotherapy.
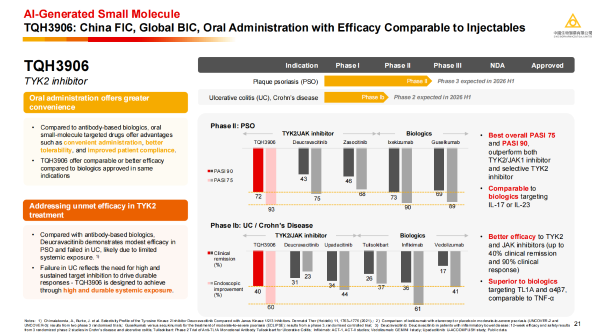
· TQH3906: A new-generation oral TYK2 inhibitor. In a Phase 2 study for plaque psoriasis, it achieved PASI-75 and PASI-90 response rates of up to 93% and 72%, respectively, comparable to biologics. In Phase 1b data for ulcerative colitis, it demonstrated a clinical remission rate of 40%, surpassing similar drugs. The company plans to initiate a Phase 3 head-to-head study against an existing TYK2 inhibitor for the treatment of psoriasis in the first half of 2026. A Phase 2 study for ulcerative colitis will also be conducted concurrently.
· TRD205: A non-opiate, highly selective antagonist targeting the AT2R receptor, has entered Phase 2 clinical trials for the treatment of acute postoperative pain and chronic pain, and is expected to become a blockbuster drug in the field of neuropathic pain.
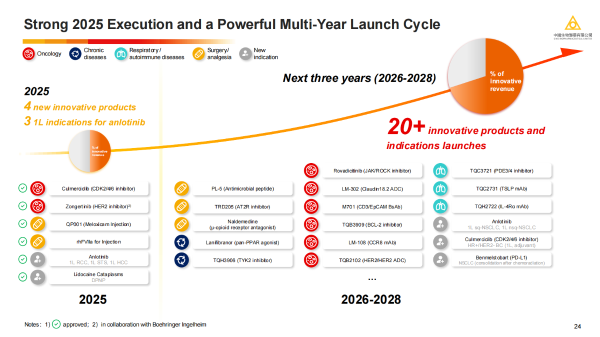
Relying on its strong pipeline portfolio and technology platforms, Sino Biopharm is entering a new stage of development driven by innovation. Between 2026 and 2028, more than 20 innovative products/new indications are expected to be approved for marketing. With the deep integration of Hygieia and LaNova Medicines, and the continuous output from internal R&D platforms, Sino Biopharm is expected to achieve a comprehensive leap in innovation quality, global layout, and long-term growth momentum, steadily moving towards its goal of becoming a leading global innovation-driven pharmaceutical company.





