
News
Sino Biopharm Ranks Third on "China's Top 100 Pharmaceutical Innovation Enterprises" List for the Seventh Consecutive Year, Remaining in the First Tier
Release Date: 2025-10-24
On October 20, the "2025 China's Top 100 Pharmaceutical Innovation Enterprises" list was released. Sino Biopharm (1177.HK) once again ranks the third. Since the list was first published in 2019, Sino Biopharm has been ranked in the first tier for seven consecutive years, reflecting Sino Biopharm's excellent strategic focus and sustained innovation momentum in a complex and ever-changing industrial environment. The list is compiled by Healthcare Executive based on a "Three Dimensions, Four Indicators" evaluation system, and is comprehensively assessed using data from Clarivate's Cortellis Competitive Intelligence, clinical trial data, and Derwent Innovation patent data.
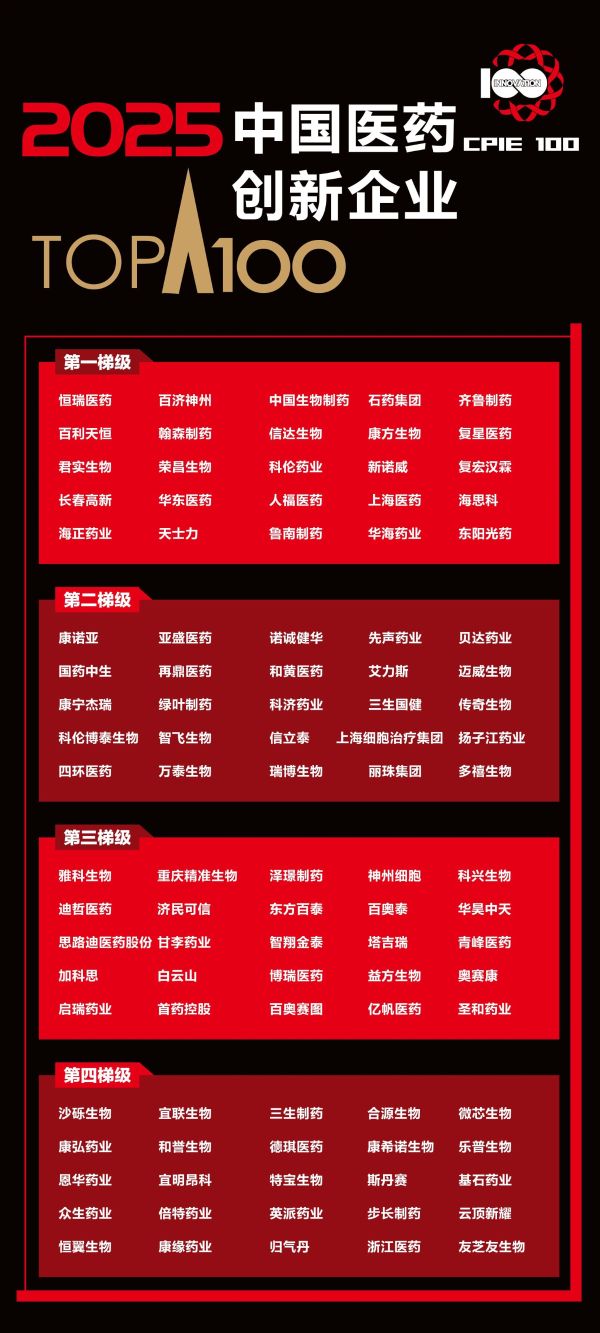
Behind the ranking is Sino Biopharm's solid performance and firm commitment to R&D investment. According to the 2025 first-half financial report, Sino Biopharm's revenue reached 17.57 billion yuan, a year-on-year increase of 10.7%. The proportion of revenue from innovative products rapidly climbed to 44.4%, laying a key foundation for achieving the goal of "innovative revenue accounting for over half of the annual total". This achievement is due to the company's continuous increase in R&D investment. In the first half of the year, R&D investment accounted for 18.1% of revenue, with nearly 70 innovative drug candidates in the clinical development stage or beyond in the four core areas.
With a dense pipeline of innovation outcomes, Sino Biopharm had several "first-of-their-kind" products approved for marketing in the first half of 2025, including Putanning®, China's first and the world's only commercially available long-acting analgesic NSAID injection; Anqixin®, China's first approved domestically produced recombinant human coagulation factor VIIa biologic; and Zongertinib (Hernexeos®), the world's first oral targeted drug for HER2-mutant advanced non-small cell lung cancer, jointly promoted in China by Sino Biopharm and Boehringer Ingelheim.
In addition to internal R&D and international cooperation, Sino Biopharm also accelerates its innovation layout through M&A. In July 2025, Sino Biopharm announced the wholly-owned acquisition of LaNova Medicines Technology (Shanghai) Co., Ltd. for a net consideration of US$500 million. LaNova Medicines is a leading R&D company in the fields of neoplasm immunity and the tumor microenvironment. This acquisition will significantly enhance Sino Biopharm's core competitiveness and international influence in neoplasm innovation, marking a key step for Sino Biopharm towards becoming a world-class innovative pharmaceutical enterprise.
Looking ahead, Sino Biopharm's innovation momentum remains strong. It is expected that nearly 20 innovative products will be approved for marketing between 2025 and 2027. Among them, more than half are expected to have peak sales exceeding RMB 2 billion, including the potential Best-in-Class (BIC) TQB3616 (a CDK2/4/6 inhibitor) and TQB2102 (an HER2 bispecific ADC) in the breast cancer field; TQC3721 (a PDE3/4 inhibitor), a potential new cornerstone drug for Chronic Obstructive Pulmonary Disease (COPD) and the first to be approved in China; and Lanifibranor (a pan-PPAR agonist), a potential first-to-market oral drug for Metabolic dysfunction-associated steatohepatitis (MASH) in China.
By the end of 2027, the number of marketed innovative products of Sino Biopharm is expected to exceed 35, with revenue from innovative products accounting for over 60% of the total. Sino Biopharm stated that in the future, it will continue to focus on the four major therapeutic fields: oncology, hepatology, respiratory, and surgery/analgesic, improve R&D efficiency and quality, and accelerate its internationalization pace to drive sustained rapid business development and steady performance growth through innovation.
Disclaimer:
1. This press release is intended to facilitate the communication and exchange of medical information and is for reference by healthcare professionals only. It is not for advertising purposes.
2. The company does not recommend any drugs and/or indications.
3. The information contained in this press release is for reference only and cannot replace professional medical guidance in any way, nor should it be considered as a diagnosis or treatment recommendation. If you wish to understand specific information about disease diagnosis and treatment, please follow the advice or guidance of a doctor or other healthcare professional.
Source: Healthcare Executive, Sino Biopharm 2025 Interim Report
- Previous:Ms. Theresa Tse Invited to Attend 2025 International Biomedical Industry Innovation Conference Beijing Forum,Sino Biopharm's Frontier Technology Innovation R&D Center to be Established in International Pharmaceutical Innovation Park
- Next:Sino Biopharm Appears at International Biopharma Industry Week Shanghai 2025, Eric Tse: Deeply Rooting and Broadly Connecting by Leveraging Shanghai as a Global Hub





